Our product portfolio includes a comprehensive range of rapid diagnostic tests, serological reagents, and biochemistry kits designed to support accurate and reliable laboratory testing. These products are developed using validated technologies and are manufactured under strict quality control systems to ensure consistent performance and reproducibility. Enison’s solutions are suitable for routine laboratory and point-of-care applications, addressing the needs
of healthcare providers and diagnostic laboratories. Please get in touch with us for detailed catalogs and technical specifications
Rapid Diagnostic Test Kits
Enison rapid diagnostic test kits are designed for the qualitative detection of specific biomarkers, providing fast and reliable results to support timely clinical decision-making
These tests are developed using validated technologies and manufactured under strict quality control systems to ensure accuracy, consistency, and ease of use. Enison rapid tests are suitable for laboratory and point-of-care applications, offering an efficient solution for screening and diagnostic purposes
By combining scientific expertise with rigorous quality standards, Enison aims to deliver diagnostic products that contribute to improved healthcare outcomes and public health monitoring
Pregnancy Rapid Test
The Enison Pregnancy Rapid Test is a qualitative in vitro diagnostic device designed for the detection of human chorionic gonadotropin (hCG) in urine samples, which aids in the early identification of pregnancy. This test is based on immunochromatographic technology and provides rapid and easy-to-interpret results. It is suitable for use in laboratory and point-of-care settings, offering a reliable screening tool when timely results are required. Manufactured under strict quality control systems, the Enison Pregnancy Rapid Test is developed to ensure consistent performance, accuracy, and ease of use in routine diagnostic application






LH Rapid Test (Ovulation Test)
The Enison LH Rapid Test is a qualitative in vitro diagnostic device designed for the detection of luteinizing hormone (LH) in urine samples, which assists in identifying the LH surge associated with ovulation. This test is based on immunochromatographic technology and provides rapid and easy-to-interpret results. It is suitable for laboratory and point-of-care applications, offering a reliable tool for ovulation prediction and fertility monitoring
Manufactured under strict quality control systems, the Enison LH Rapid test is developed to ensure consistent performance, accuracy, and ease of use in routine diagnostic applications
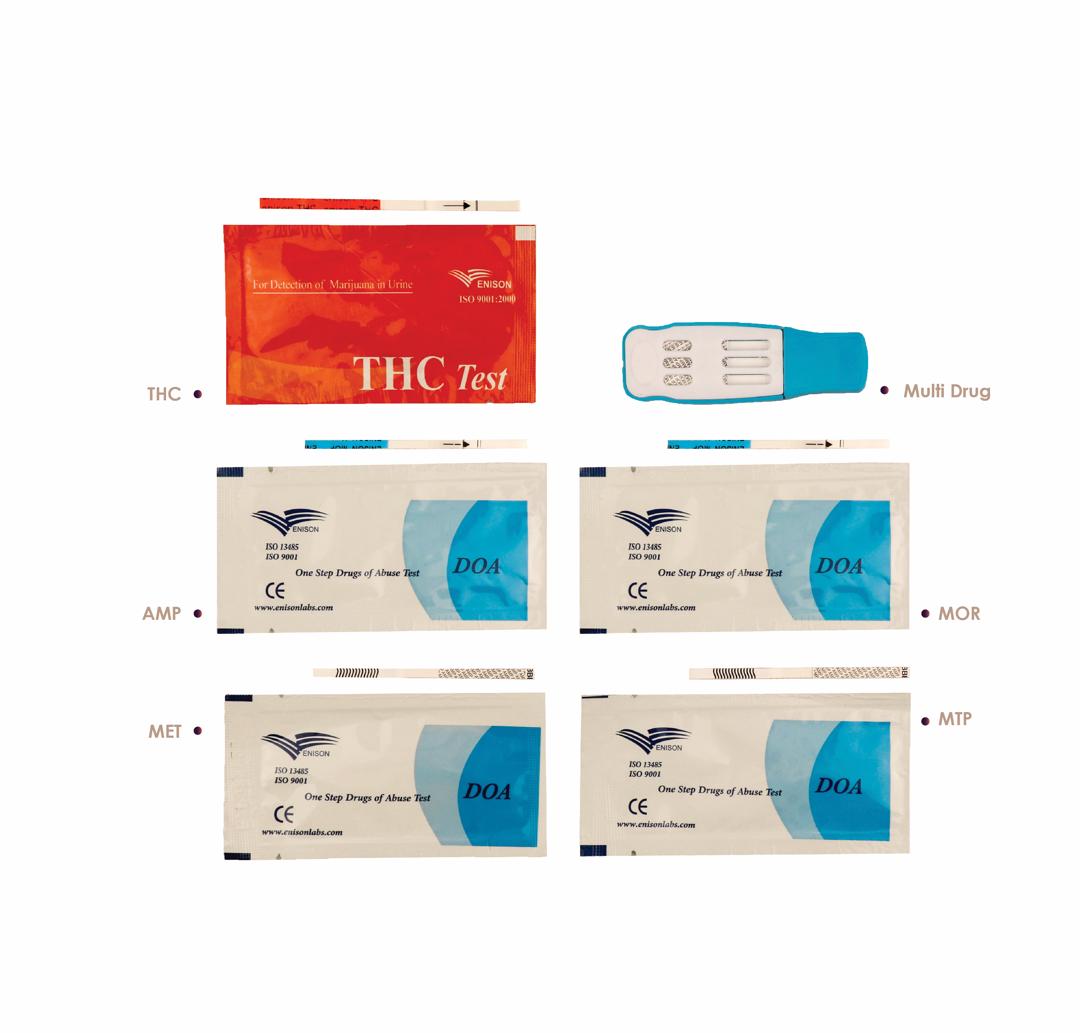
Rapid Drug Abuse Tests
Enison Rapid Drug Abuse Tests are qualitative in vitro diagnostic devices designed for the detection of drugs of abuse and their metabolites in human urine samples.
These tests are based on immunochromatographic technology and provide rapid, reliable, and easy-to-interpret results, making them suitable for laboratory and point-of-care applications. They are intended for screening purposes and support timely decision-making in various testing environments. Manufactured under strict quality control systems, Enison drug abuse rapid tests are developed to ensure consistent performance, accuracy, and ease of use, in accordance with applicable quality and regulatory standard
Gold Nanoparticles
Gold nanoparticles synthesized at Enison are developed using controlled and optimized chemical methods to achieve consistent particle size, stability, and surface properties. These nanoparticles are specifically designed for use in diagnostic applications, including rapid immunochromatographic tests, where they play a critical role as signal-generating labels. The synthesis process is carefully monitored to ensure reproducibility, batch-to-batch Consistency, and compatibility with biological molecules such as antibodies and antigens. Through in-house research and development, Enison focuses on producing gold nanoparticles with reliable performance, contributing to the accuracy, sensitivity, and overall quality of diagnostic test systems
CRP Serology Kits
Enison CRP Serology Kits are in vitro diagnostic devices designed for the qualitative and semi-quantitative detection of C-reactive protein (CRP) in human serum samples. These kits are designed to support the assessment of inflammatory conditions, based on principles of immunological reactions to ensure reliable and reproducible results. Enison CRP serology products are suitable for routine laboratory use and are developed to provide consistent performance under standard testing conditions. Manufactured in compliance with strict quality control systems, Enison CRP kits are designed to meet recognized laboratory and diagnostic standards, supporting accurate and dependable serological testing
